Caution: The following texts are translated from the Japanese original. We cannot take responsibility for ambiguity in the translation.
Designated Category 2 OTC medicine
ジーフォーL注入軟膏 G4L INJECTION OINTMENT
Product features
■ G4-L injection ointment...
● Contains prednisolone acetate, which reduces itching, swelling and bleeding in the affected area.
● Can be applied and injected directly into the affected area without getting your fingers dirty.
Indication
To alleviate pain/bleeding/swelling/itching due to bleeding piles/blind piles
Dosage and administration
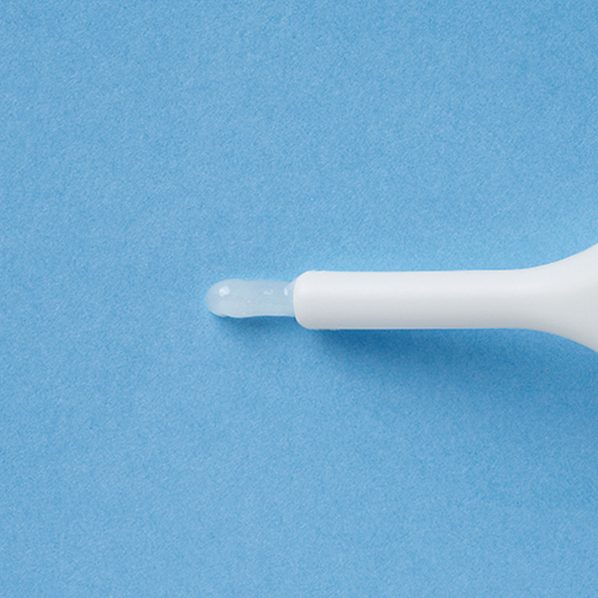
<When injected into the anus>
Insert the nozzle part into the anus and slowly inject the entire volume.
[Age: single dose: number of daily doses]
Adult (15 years old and over): 1pieces: 1 to 2 times
Under 15 years old: Do not use
<When applied to the anus>
Apply the following amount to the anus. Do not use the used for application for injection use.
[Age: single dose: number of daily doses]
Adult (15 years old and over): appropriate amount: 1 to 3 times
Under 15 years old: Do not use
Precautions of Dosage and Administration
(1)Strictly follow the prescribed dosage and administration.
(2)Do not let children use.
(3)Use the medicine only in the anus.
Ingredient and amount
2grams in 1pieces
Prednisolone acetate:1mg:Reduces itching and swelling in the affected area.
Lidocaine:60mg:Reduces pain and itching in the affected area.
Allantoin:20mg:Helps repair mucous membranes.
Tocopherol acetate:50mg:Promotes blood circulation, improves blood congestion in the affected area.
Precautions
■When not to use the product
(If you do not follow these instructions, the current symptoms may worsen or adverse reactions are more likely to occur.)
1.This product should not be used by the following persons
(1) Patients who have had an allergic symptom to this drug or its ingredients.
(2) Patients with purulent hemorrhoidal lesions.
2.Do not take this medicine for a long time
■Consultation
1.The following persons should contact a physician, pharmacist, or registered salesperson for a consultation before use.
(1)Patients undergoing medical treatment from a physician
(2)Pregnant women or women suspected of being pregnant
(3) Patients who have experienced allergic symptoms associated with drugs, etc.
2.If the following symptoms are observed after using this drug, these may be adverse reactions, so immediately discontinue the use, and show this document to your physician, pharmacist, or registered sales person for a consultation.
Skin:rash/redness,itching,swelling
Other:irritation,purulence
The following serious symptoms may occur in rare cases. In such cases, immediately seek medical aid:
<Symptom name: Symptoms>
Shock (anaphylaxis):Symptoms, such as itching of skin, urticaria, hoarseness, sneezing, itchy throat, breathing difficulties, palpitations, and clouding of consciousness may occur immediately after use.
3.When symptoms do not improve even after using this medicine for about 10 days, stop taking this medicine and consult a physician, pharmacist or registered salesperson, being sure to take this instruction leaflet with you.
Additive
Liquid paraffin, glyceryl monostearate, condensed polyglyceryl ricinoleate, white petrolatum
Precautions for storage and handling
(1) Store in a cool, low humidity place away from direct sunlight.
(2)Keep out of reach of children.
(3)Do not transfer the medicine to a different container. (It may lead to misuse or alter the quality of the drug. )
(4)Do not use products that have expired.
(5)Do not flush used containers into the toilet.
Disclaimer on Multilingual Product Information
・This product is a pharmaceutical product approved under a Japanese law, the Law for Ensuring the Quality, Efficacy and Safety of Drugs and Medical Devices, with a view to its sale and use in Japan.
・Multilingual product information is a translation of the product labeling written in Japanese and provided for your information only. It does not warrant that its contents and the product itself conforms to laws and regulations in countries other than Japan.
・Multilingual product information is a tentative translation by Our Company, and may be modified or altered without notice.
・Our Company assumes no responsibility for any occurred problem attributable to the contents of the multilingual product information.